Improve Quality, Stay Compliant, Be Efficient!
Optimize document control, audit management, CAPA tracking, and quality event handling with an automated eQMS. Ensure regulatory compliance with precision, efficiency, and real-time oversight.
GARTNER® recognized
G2 favorite
Achieve Superior Quality & Compliance
An Electronic Quality Management System (eQMS) simplifies quality control and regulatory compliance, ensuring seamless documentation management and adherence to industry standards.
Quality Management Excellence
Enhance operational efficiency with digitally optimized quality control systems, offering automated compliance tracking and real-time oversight for continuous improvement.
Compliance Assurance
Effortlessly meet regulatory requirements and improve audit readiness with a centralized document control system and eQMS automation.
Process Optimization & Efficiency
Maximize productivity through streamlined digital workflows, ensuring error-proof documentation, process standardization, and improved operational efficiency.

DIGITAL IMPROVEMENT
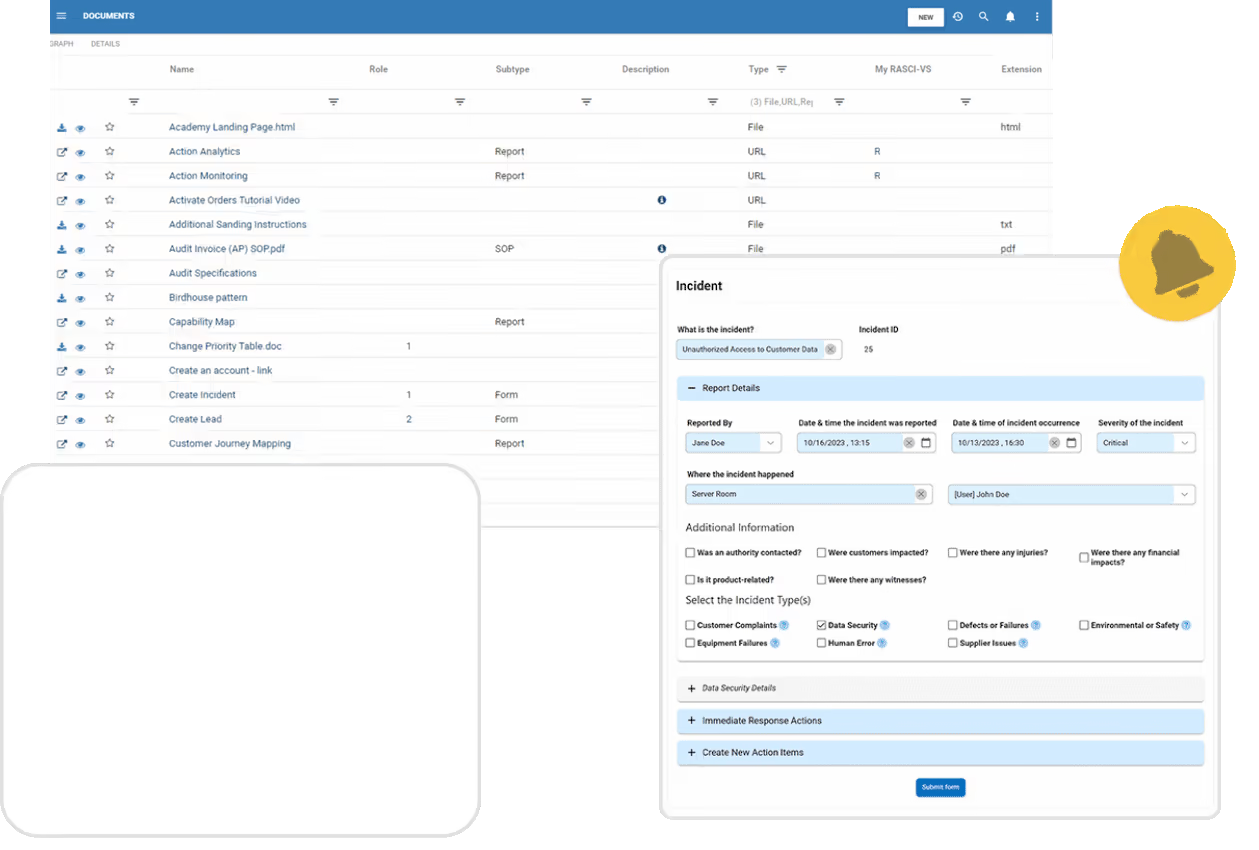
Enhanced Information & Document Control
Effortlessly manage content lifecycle, assign ownership, and maintain document governance with structured approval workflows and change request tracking. Ensure regulatory adherence with secure digital signatures and audit-ready records.
Content
Simplify document control by automating approvals, ensuring version control, and enforcing compliance-ready governance for seamless information management.
Signatures
Protect document integrity with encrypted digital signatures, providing time-stamped authentication for audit trails and long-term regulatory compliance.
New Gen eQMS Software
Learn what separates eQMS from the commonly available options
Common QMS Issues
- Limited Traceability of processes & documentation
- too many progress check meetings for all attendees
- Access to paper archive is a challenge
- Complex process to complete accurate risk management and risk analysis
- High risk of lost or misfiled documentation
- Out of sync or obsolete records in circulation
- Missing signatures during an audit are a high risk
- Lack of accountability and visibility within teams
- Inefficiency and mistakes lead to high cost to maintain quality
eQMS Advantage
- Detailed log kept of all revisions, signatures, and updates, stored in a secure central repository
- Change & request notifications to specific personnel sent automatically
- 24/7 access from any place to all documents & data
- Immediate access and traceability of all Risk management files, reports & SOPs
- Electronic document control with API integration on Cloud-based data centers
- Document control in place with status and versioning
- Any unsigned document would be automatically flagged
- Log or revisions, dates, and employees who authored or reviewed the documents
industry recognized
Build Company Transparency
Gain full visibility into the usage and applicability of records. Maintain digital content with clear accountability, including roles and responsibilities.
Ensure Transparency
Gain real-time insights into document usage and applicability, ensuring accurate version control, audit readiness, and seamless compliance tracking.
Conduct Impact Analysis
Assess downstream effects on policies, SOPs, business units, and related records. Ensure proactive risk management and data-driven decision-making with comprehensive change impact assessments.

Get Started Today!
Enhance system oversight and document control with a powerful eQMS solution. Streamline workflows, boost compliance efficiency, and drive operational excellence with the right digital transformation!
Frequently Asked Questions
Most frequent questions and answers
What is eQMS?
eQMS, or Enterprise Quality Management Software, is a centralized digital solution that helps organizations streamline quality processes, ensure regulatory compliance, and improve operational efficiency. It supports document control, audit management, CAPA, and risk assessment to maintain high industry standards.
How can eQMS benefit my organization?
Implementing an eQMS enhances efficiency and compliance, reduces risk exposure, and ensures quality consistency. It enables automated workflows, real-time data insights, and audit readiness, leading to better decision-making and customer satisfaction.
Is eQMS suitable for my industry?
Yes, eQMS solutions are highly adaptable and cater to industries such as manufacturing, healthcare, pharmaceuticals, food & beverage, and more. The system can be configured to meet specific regulatory and operational needs.
Is your eQMS software compliant with industry standards and regulations?
Absolutely. Our eQMS solution supports compliance with ISO, FDA, and other global regulations, helping businesses maintain audit-ready records, enforce standard operating procedures (SOPs), and meet quality assurance requirements.
Can eQMS integrate with other systems?
Yes, our eQMS seamlessly integrates with ERP, CRM, and other enterprise software, ensuring a connected workflow across departments for improved data management and process automation.
How easy is it to implement eQMS in my organization?
Our intuitive platform ensures a smooth onboarding process with dedicated implementation support. We provide training, customization, and expert guidance to help your organization transition efficiently to a fully digital quality management system.
Still have Questions?
Please send us a message on the designated page or book a demo to speak to us directly in person!
More About Us
At Interfacing, our dedicated team of experts delivers innovative eQMS solutions designed to streamline quality management and ensure regulatory compliance across industries. Our software enhances efficiency, process automation, and data-driven decision-making, making it the ideal choice for businesses committed to excellence in quality and risk management.

